Organization chart
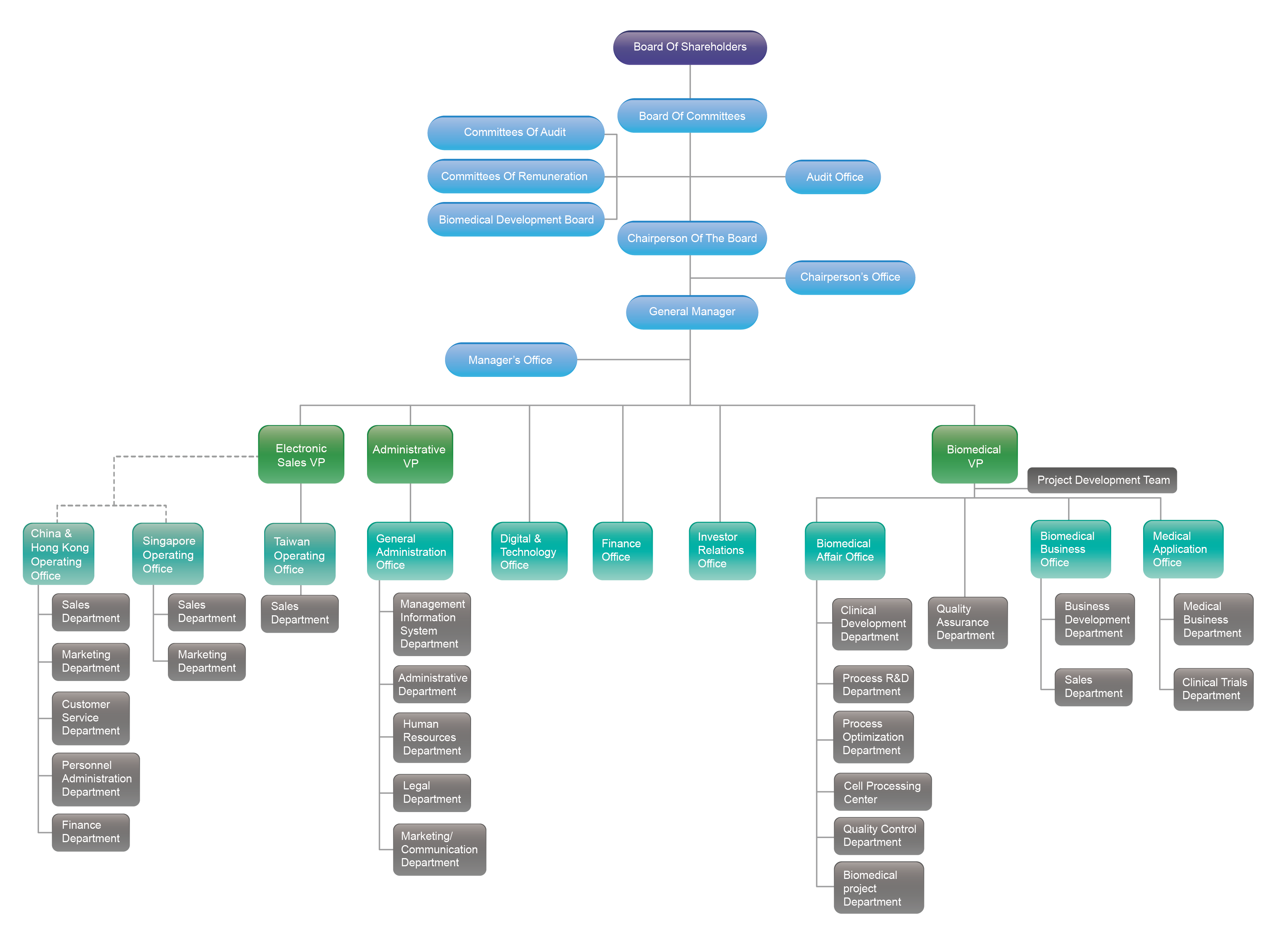
Heads of departments
- Audit Office
-
- Assist the Board of Directors and managers to check and review the lack of internal control, to measure the effectiveness and efficiency of operations, and to provide the improvement suggestions in a timely manner.
- Responsible for the audit and tracking of various operating cycles.
- Chairman’s Office
-
- In order to assist the Chairman in planning the Conglomerate’s important policies, guidelines and improvement countermeasures, a specific group responsible may be set up if necessary.
- Responsible for assisting the chairman or independent directors in stock affairs- related matters such as convening the meetings, contacting, sending, and writing various proposals (e.g. Board of Directors, Remuneration Committee, Audit Committee, shareholders’ (or temporary) meeting).
- Responsible for assisting the Chairman and Directors in communication and coordination between various departments and performing assigned tasks.
- President’s Office
-
- Responsible for formulation, evaluation and implementation of various projects throughout the company.
- Responsible for the coordination and system planning of various departments.
-
General
Management Office -
Ordinate and control the human resources, legal affairs, administration, general affairs, marketing, procurement (except for electronic business-related products),
- Information
Department Responsible for the planning, construction and maintenance of the informationization, computerization, information integration, and information security management of the operating systems in the departments of the Conglomerate.
- Management
Department -
- Responsible for the Conglomerate’s general affairs and marketing related matters.
- Responsible for the procurement and management of the Conglomerate’s fixed assets, internal and external items and equipment (except for electronic business- related commodities).
- Responsible for the release of funds within the Conglomerate.
- Human Resources Department
-
- Responsible for the personnel recruitment and allocation, personnel training and development, human resource planning, remuneration management, performance management, labor relations management and administrative operation management within the Conglomerate.
- Responsible for the review of salaries and bonuses within the Conglomerate.
- Legal Affair Department
Responsible for the formulation of measures related to corporate governance, interpretation of regulations, legal affairs, and litigation within the Conglomerate.
- Planning Department
-
- Responsible for the collection, integration and market analysis of industrial information.
- Responsible for the planning, design, marketing and maintenance of the company’s external websites.
- Responsible for the product functions, market positioning, business policies, strategy formulation, and cross-departmental communication and coordination.
- Responsible for the product policy, marketing plan, advertising planning, marketing channel integration, marketing strategy planning and promotion.
- Finance Department
-
- Responsible for the provision and analysis of financial management information within the Conglomerate.
- Responsible for financial scheduling, capital management and other business within the Conglomerate.
- Responsible for the Conglomerate’s accounting processing, statement preparation and tax planning.
- Responsible for the compilation and management of the Conglomerate’s budget.
- Responsible for the internal stock affairs operations, shareholders’ meetings, Board of Directors, functional committees, and corporate governance-related matters in the Conglomerate.
- Digital&Technology Office
-
- Responsible for digitalization of laboratory and clinical data, etc.
- Introducing AI technology and application programs in the future.
- Investor Relations Office
-
- Construct the interactive relationship between the company and natural persons, legal persons, the media, and the authorities.
- Assist the company’s financial, business and other departments in expanding the public relations.
- Plan and hold the investor conference, and assist in press conferences and other matters.
- Responsible for the company’s public speaking.
Electronics Business Group
Responsible for managing related affairs of the operations office in Taiwan, China, Hong Kong, and Singapore.
- Taiwan Operations Office
-
Responsible for coordinating the electronic operations in Taiwan.
- Sales Department
-
- Responsible for the procurement and planning of all electronic product lines.
- Responsible for the market research, research and analysis, product sales plan formulation and solving customer product design and process problems in electronic product
- Responsible for the operation and management of electronic commodity within the Conglomerate, such as in inventory, shipment, and so on.
- Responsible for the collection and progress tracking of accounts receivable.
-
China and Hong Kong
Operations Office -
Responsible for coordinating the electronic operations in China and Hong Kong.
- Singapore Operations Office
-
Responsible for coordinating the electronic operations in Singapore and Southeast Asia.
Biomedical Business Group
Responsible for the management of related affairs of the Biomedical Business Division and the Medical Application Office.
- Project Management Team
-
- Project Management: For various projects such as government projects, internal projects, external cooperation projects, award applications, and so on. The R&D cycle shall be introduced in accordance with the company’s relevant management regulation, and the progress of the project shall be controlled.
- Intellectual Property Management: Realize the outcome of R&D in different forms, such as patent application and maintenance, academic journals publication, trade secrets, copyrights, and management of other publications.
- Quality Assurance Department
-
- Quality Assurance: Implement the quality system and verification operation management of the factory that complies with pharmaceutical regulations; establish and implement the audit/inspection operations and follow-up correction and prevention in line with pharmaceutical regulations; the training and management of quality system personnel in the factory, and the formulation and maintenance of quality procedures and other quality systematic activities and standards, so that products and services can meet the quality requirements.
- Quality Document Management: Comprehensively manage the document control and archiving management operations of the factory quality system.
- Biomedical Division
-
- Responsible for coordinating the product research and development and technological development related to the biomedical business.
- Responsible for coordinating the construction, maintenance and management of the cell process center.
- Responsible for coordinating the application and implementation of clinical trials and Regulations Governing Specific Medical Techniques.
- Responsible for coordinating cooperation matters between domestic hospitals.
- Responsible for coordinating the marketing and sales of the biomedical products.
- Responsible for coordinating the testing and management of the quality control related to biomedical.
- Responsible for coordinating the issuance, distribution, archiving and management of quality documents related to biomedical
- Clinical Department
-
- Medical affairs/collection, presentation and training of medical and scientific information/assistance in clinical development/establishment and maintenance of KOL relationships/market analysis.
- Process R&D Department
R&D: Comprehensively manage the research and development of the process and standardization operations, including the new technology research and process introduction, to conduct the pilot production operations; optimize the current process to reduce the raw material consumption; and analyze and evaluate the process cost of new cases.
- Process Optimization Department
Mainly responsible for standardization and optimization of existing formulation processes. Focusing on sheet technology.
- Cell Preparation Center
-
- Factory Affairs Division: Comprehensively manage the operations related to facility and the equipment maintenance, and setup the verification in the factory.
- Manufacturing Division: Manage the manufacturing-related operations in the factory.
- Production Management Division: Comprehensively manage the management of production scheduling and planning in the factory, including the formulation, maintenance and follow-up of production schedule; confirmation of orders; maintenance of order shipments; coordination and communication; and the operation related to raw material warehousing and inventory management.
- Quality Control Department
-
- Quality Control Division: Manage related testing operations in the quality system of the factory, including incoming quality control (IQC), incoming process quality control (IPQC), final product quality control (FQC) and other inspection project management; inspection platform maintenance and development; report issuance & management; quality control system maintenance; traceability operations.
- Quality Testing Laboratory: Operate in accordance with ISO/IEC 17025: 2017 compliance certification laboratory, including specimen collection, inspection on quality control, on-board operation, result review, and report preparation.
- Biomedical project Department
-
Responsible for analyzing, evaluating and implementing specific projects in the biomedicine department, such as cell banks and tissue banks.
- Biomedical Business Office
Coordinating the marketing, promotion and sales of products in the biomedical business department.
- Business Development Department
Responsible for marketing planning/strategy formulation, implementation of marketing activities, and support of business units.
- Sales Department
-
- Sales promotion
- Business development
- Responsible for planning and implementing business plans and achieving performance goals.
- Medical Application Office
-
- Responsible for coordinating the development assessment of the company’s new medical business and the clinical development of new applications.
- Responsible for domestic and foreign business development as well as the planning and introduction of the company’s cooperation model.
- Responsible for coordinating the cooperation and strategic development of domestic biotechnology-related government agencies and legal persons.
- Clinical Trial Department
-
- Responsible for collecting and evaluating the development of new products and new clinical applications of existing products.
- Responsible for collecting and discussing with specialists to evaluate the feasibility and introduction of products in clinical.
- Responsible for the follow-up tracking and management of new products and existing products after the real application in the hospitals.
- Medical Development Department
-
- Assist in promoting related matters such as information collection and production of evaluation reports by the biotechnology team.
- Responsible for the on-marketing plan of regenerative medicine products.
- Responsible for the evaluation, introduction and follow-up promotion of foreign regenerative medicine products and medical technologies in oversea
- Responsible for collecting the latest technologies, patents, information of domestic and foreign regenerative medicine; and analyze and evaluate the related company data.
- Responsible for connecting the domestic government agencies and related legal persons to establish a strategic alliance for regenerative medicine through cooperation and to deepen the domestic regenerative medicine industry.